-
Beyond Air® Presents Positive Study Update from the At-Home LungFit® GO Pilot Study for Nontuberculous Mycobacterial Lung Disease at the American Thoracic Society International Conference 2022
来源: Nasdaq GlobeNewswire / 17 5月 2022 15:05:00 America/Chicago
A total of 2,323 inhalations have been self-administered at home to date with no treatment related discontinuations reported and overall high treatment compliance
All subjects have successfully titrated to receive the target 250 ppm NO regimen at home
LungFit® GO is the first NO generator and delivery system safely used in a clinical trial in the home setting with patients self-administering high concentration NO treatment
GARDEN CITY, N.Y., May 17, 2022 (GLOBE NEWSWIRE) -- Beyond Air, Inc. (NASDAQ: XAIR), a clinical-stage medical device and biopharmaceutical company focused on developing inhaled nitric oxide (NO) for the treatment of patients with respiratory conditions, including serious lung infections and pulmonary hypertension and, through its affiliate Beyond Cancer, ultra-high concentration nitric oxide (UNO) for the treatment of solid tumors, today announced positive new interim data from the ongoing LungFit® GO pilot at-home study in Australia.
In this study, patients self-administered high concentration inhaled NO at home to treat severe nontuberculous mycobacterial (NTM) lung disease. These data were included in a poster presentation at the American Thoracic Society International Conference 2022 (ATS 2022), which is being held in San Francisco from May 13-18, 2022. The Company expects complete safety and efficacy results to be reported later in 2022.
At the time of the data cutoff on April 4, 2022, a total of 15 subjects were enrolled in the pilot study. The mean age of subjects was 62.1 years (range: 22–82 years) with the majority female (80%), a distribution consistent with real-world NTM disease.
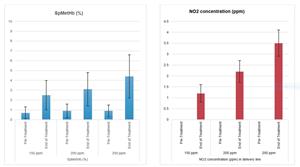
The data show that high concentration inhaled NO was well tolerated following a total of 2,323 inhalations self-administered at home with no treatment related discontinuations reported and overall high treatment compliance. All 15 subjects were successfully titrated to 250 ppm NO in the hospital setting, and none have required dose reductions during the subsequent at-home portion of the study. Methemoglobin and NO2 concentrations remained within acceptable ranges in all subjects during NO treatment, below the safety thresholds of 10% and 5 ppm, respectively. Patients are followed up for 12 weeks after the 12-week treatment period is completed and the last patient visit at the end of week 24 will occur in August 2022. The totality of the data will be used to evaluate efficacy measures, including quality of life, physical function, and sputum bacteria as compared to baseline measurements.
“The LungFit Go pilot study has provided data that opens the door to treating patients suffering from NTM lung infection, as well as other chronic, refractory lung infections, with high concentration NO in the comfort of their own homes or anywhere there is electric power. Most importantly, in addition to the strong safety and tolerability data, patients reported quality-of-life benefits from receiving the home NO treatment in the majority of quality-of-life endpoints, with the most improved benefits being NTM symptoms and digestive symptoms,” said Steve Lisi, Chairman and Chief Executive Officer of Beyond Air.
Adverse Events Total N (ITT** Population) = 15 N % Any AE 14 93.3 Any AE related to study treatment * 9 60.0 Any AE related to study treatment classified as Severe * 0 0 Any Serious AE (SAE) (SAE)6 40.0 Any Treatment Emergent SAE occurring during treatment period 2 13.3 Any SAE related to study treatment * 1 6.7 *Including possibly, probably, and definitely related
**Intent to treat“The LungFit is an impressive device that may allow for the use of nitric oxide in the home setting for NTM patients. These data provide hope for these severely sick patients with limited therapeutic options, that there can be a therapy with a favorable safety and tolerability profile which will improve quality of life and may have an impact on bacteria,” stated Dr. Rachel Thomson MBBS, PhD, FRACP; Professor at University of Queensland, School of Medicine.
Dr. Thomson presented the interim results at the ATS 2022 in poster titled, “Home-based Intermittent Inhaled High-Dose Nitric Oxide (NO) in Nontuberculous Mycobacterial Pulmonary Disease Using a Novel NO Generator and Delivery System is Safe and Well-Tolerated.” The poster is available on the Publications page of the Beyond Air website (click here).
LungFit® GO NTM Trial Design
The 12-week, multi-center, open-label clinical trial is supported by the US Cystic Fibrosis Foundation and taking place in Australia for adult subjects with chronic refractory NTM lung disease. The trial enrolled both cystic fibrosis (CF) and non-CF subjects chronically infected with Mycobacterium avium complex (MAC), Mycobacterium abscessus complex (MABSC) or other strains of NTM who are refractory to standard therapies. The trial consists of a run-in period followed by two treatment phases. The run-in period provides a baseline for the efficacy endpoints, such as patient physical function and bacterial load. The first treatment phase takes place over a two-week period and begins in the hospital setting where subjects are titrated from 150 ppm NO up to 250 ppm NO over several days. During this first treatment phase subjects receive NO for 40 minutes, four times per day while methemoglobin and nitrogen dioxide (NO2) levels are monitored. Subjects are trained to use LungFit® GO and subsequently discharged to complete the remaining portion of this two-week treatment period at their home continuing the established highest tolerated NO concentration. For the second treatment phase, a 10-week maintenance phase, the inhalation treatments are administered twice daily at the same NO level. Subjects are evaluated for an additional 12 weeks after the end of treatment. The study evaluates safety, tolerability, quality of life, physical function, and bacterial load among other parameters, as compared to baseline measurements.About Beyond Air, Inc.
Beyond Air, Inc. is a clinical-stage medical device and biopharmaceutical company developing a revolutionary NO Generator and Delivery System, LungFit®, that uses NO generated from ambient air to deliver precise amounts of NO to the lungs for the potential treatment of a variety of pulmonary diseases. The LungFit® can generate up to 400 ppm of NO, for delivery either continuously or for a fixed amount of time and has the ability to either titrate dose on demand or maintain a constant dose. The Company is currently applying its therapeutic expertise to develop treatments for pulmonary hypertension in various settings, in addition to treatments for respiratory tract infections that are not effectively addressed with current standards of care. Beyond Air is currently advancing its revolutionary LungFit® for clinical trials for the treatment of severe lung infections such as acute viral pneumonia (including COVID-19) and nontuberculous mycobacteria (NTM). Additionally, Beyond Air is using ultra-high concentrations of NO with a proprietary delivery system to target certain solid tumors in the pre-clinical setting. For more information, visit www.beyondair.net.About Nitric Oxide (NO)
Nitric Oxide (NO) is a powerful molecule, naturally synthesized in the human body, proven to play a critical role in a broad array of biological functions. In the airways, NO targets the vascular smooth muscle cells that surround the small resistance arteries in the lungs. Currently, exogenous inhaled NO is used in adult respiratory distress syndrome, post certain cardiac surgeries, and persistent pulmonary hypertension of the newborn to treat hypoxemia. Additionally, NO is believed to play a key role in the innate immune system and in vitro studies suggest that NO possesses anti-microbial activity not only against common bacteria, including both gram-positive and gram-negative, but also against other diverse pathogens, including mycobacteria, viruses, fungi, yeast, and parasites, and has the potential to eliminate multi-drug resistant strains.About the LungFit® GO*
Beyond Air’s LungFit® Go is a portable device that weighs only ~20 lbs. and operates with a standard electrical outlet (120-240 volts). Since NO is generated from ambient air that flows through a reaction chamber, there is an unlimited supply. Beyond Air’s proprietary nitrogen dioxide (NO2) filters are required for the system to generate and safely deliver NO. Toxic levels of NO2 can result from high concentrations of NO without proper filtration. The filters also program the system, via an attached RFID chip, with respect to NO concentration, flow rate and duration of therapy. The Company believes this design provides maximum flexibility for NO administration. Filters are single use and there are no special requirements for disposal. Alarms monitor system performance.* Beyond Air’s LungFit® systems are not approved for commercial use. Beyond Air’s LungFit® systems are for investigational use only.
About NTM
Nontuberculous mycobacteria (NTM) infection is a rare and serious bacterial infection in the lungs causing debilitating pulmonary disease associated with high morbidity and mortality. NTM infection is acquired by inhaling aerosolized bacteria from the environment, and can lead to NTM lung disease, a progressive and chronic condition. According to the Cystic Fibrosis Foundation, 13% of U.S. cystic fibrosis patients had a positive culture for a NTM species in 2017. NTM is an emerging public health concern worldwide because of its multi-drug antibiotic resistance. Current treatment guidelines suggest a combination of multiple antibiotics dosed chronically for as long as two years. These complex, expensive and invasive regimens have a poor record in the treatment of Mycobacterium abscessus complex (MABSC) and refractory Mycobacterium avium complex (MAC) and have the potential to cause severe adverse events. Beyond Air’s system is designed to deliver 150 - 400 ppm NO to the lung, and early data indicate that this range of NO concentrations could have a positive effect on patients infected with NTM.Forward Looking Statements
This press release contains “forward-looking statements” concerning inhaled nitric-oxide and the Company’s LungFit® product, including statements with regard to potential regulatory developments and the expected timing thereof, expected product launch for the Company’s LungFit® product and the timing thereof, and the potential impact on patients and anticipated benefits associated with its use. Forward-looking statements include statements about our expectations, beliefs, or intentions regarding our product offerings, business, financial condition, results of operations, strategies or prospects. You can identify such forward-looking statements by the words “anticipates,” “expects,” “intends,” “impacts,” “plans,” “projects,” “believes,” “estimates,” “likely,” “goal,” “assumes,” “targets” and similar expressions and/or the use of future tense or conditional constructions (such as “will,” “may,” “could,” “should” and the like) and by the fact that these statements do not relate strictly to historical or current matters. Rather, forward-looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made. Because forward-looking statements relate to matters that have not yet occurred, these statements are inherently subject to risks and uncertainties that could cause our actual results to differ materially from any future results expressed or implied by the forward-looking statements. These forward-looking statements are only predictions and reflect our views as of the date they are made with respect to future events and financial performance. Many factors could cause our actual activities or results to differ materially from the activities and results anticipated in forward-looking statements, including risks related to: the potential that regulatory authorities, including the FDA and EMA, may not grant or may delay approval for our product candidate; the impact of the COVID-19 pandemic on the FDA’s review process; our approach to discover and develop novel drugs, which is unproven and may never lead to efficacious or marketable products; our ability to fund and the results of further pre-clinical and clinical trials; obtaining, maintaining and protecting intellectual property utilized by our products; our ability to enforce our patents against infringers and to defend our patent portfolio against challenges from third parties; our ability to obtain additional funding to support our business activities; our dependence on third parties for development, manufacture, marketing, sales, and distribution of products; the successful development of our product candidates, all of which are in early stages of development; obtaining regulatory approval for products; competition from others using technology similar to ours and others developing products for similar uses; our dependence on collaborators; our short operating history and other risks identified and described in more detail in the “Risk Factors” section of the Company’s most recent Annual Report on Form 10-K and other filings with the SEC, all of which are available on our website. We undertake no obligation to update, and we do not have a policy of updating or revising, these forward-looking statements, except as required by applicable law.CONTACTS:
Corey Davis, Ph.D.
LifeSci Advisors, LLC
Cdavis@lifesciadvisors.com
(212) 915-2577A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/7f55691a-f708-4b35-9150-12682076d800